ZYGOMATIC
IMPLANTS
Innovative variations
to treat the severely resorbed maxilla
Zygomatic Implants
Innovative variations
to treat the severely resorbed maxilla
The Zygomatic
implant range
Tailored implant variations, designed to address the specific anatomical needs in the diverse ZAGA classification, ensuring optimal prosthetic outcomes.
.
ZYGIN Family
Compact head design accross the range with a rescue option available.
ZYGIN-W:
The Wide ZYGIN (ZYGIN-W) implant is a rescue implant.
- Wider apical thread diameter – Ø4.20 vs Ø3.40 mm.
- Increased Thread pitch – 0.75 vs 0.60 mm.
ZYGON:
Smooth coronal region with no threads, ideal for resected and extremely atrophic maxillae.
- 0.75 mm thread pitch.
Family uses MC-ZYG (MUA)
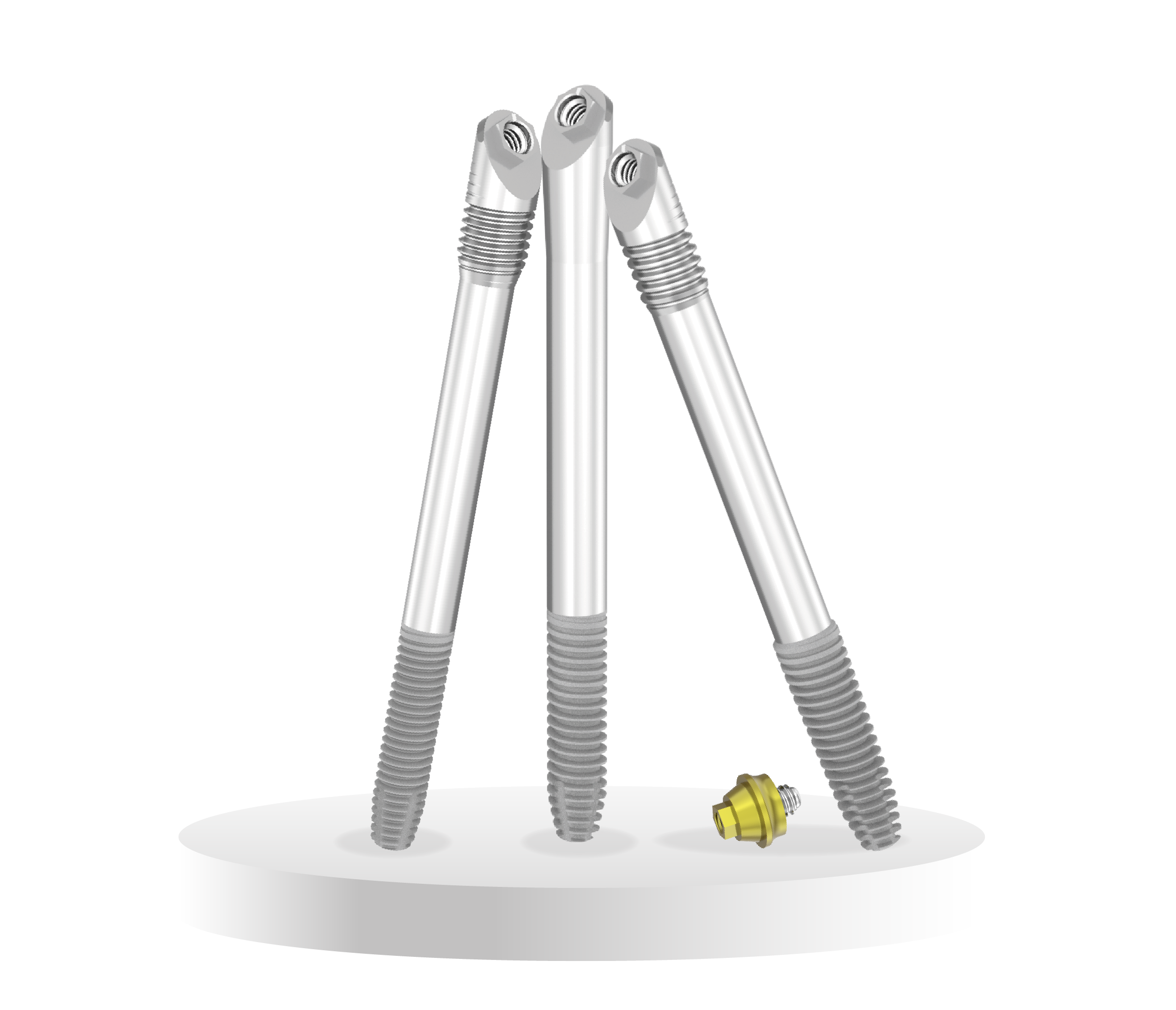
Consists of 3 variations; ZYGIN, ZYGIN-W and ZYGON.
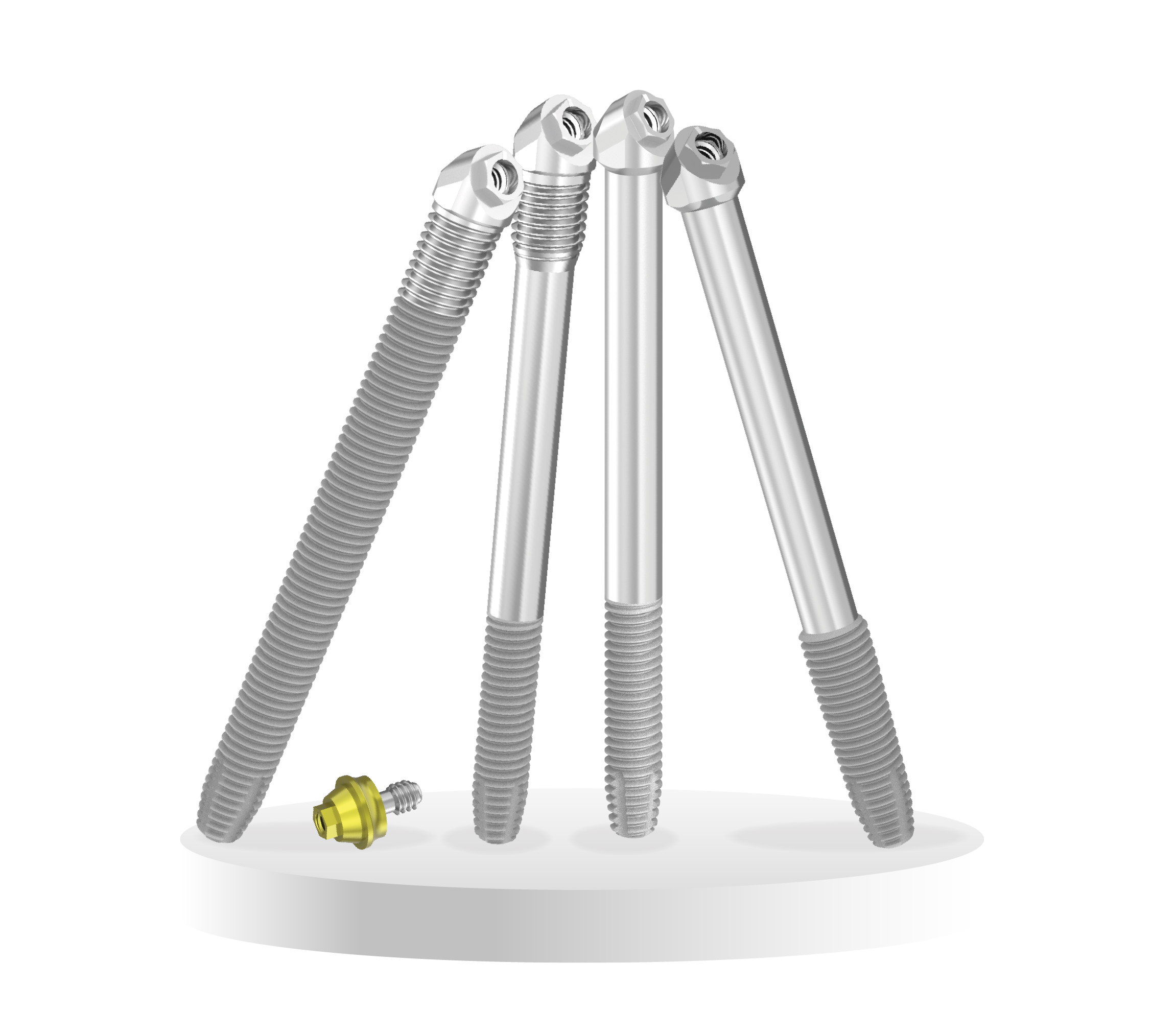
Consists of 4 variations; ZYG, ZYGAN®, ZYGEX and ONC.
Zygomatic Family
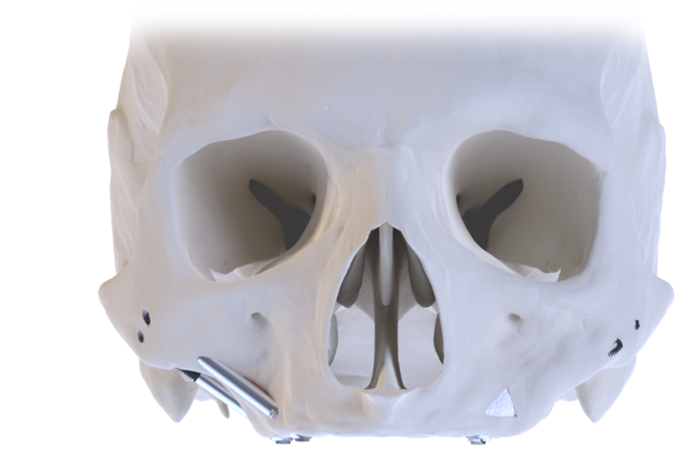
Following traumatic accidents, oncology resections or congenital defects, patients present with missing sections of bone and connecting soft tissue. These situations can pose significant physical and psychological effects on the patient.
Each Co-Axis® enabled with a 55° prosthetic angulation correction. Technique selection should consider ridge crest concavity and sinus anatomy. The ZAGA approach classifies anatomy to guide zygomatic placement. Southern’s Zygomatic range offers implant options tailored to the ZAGA classification and technique.
- External Hex Connection – More “forgiving” in situations of impassive fit or implant divergence
- Body Variations – To match the ZAGA classification and placement technique
- Narrower Apex – Ideal for patients with smaller anatomy
Family uses AMCZ (MUA)
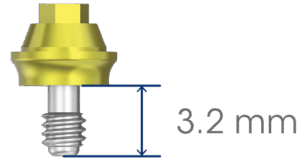
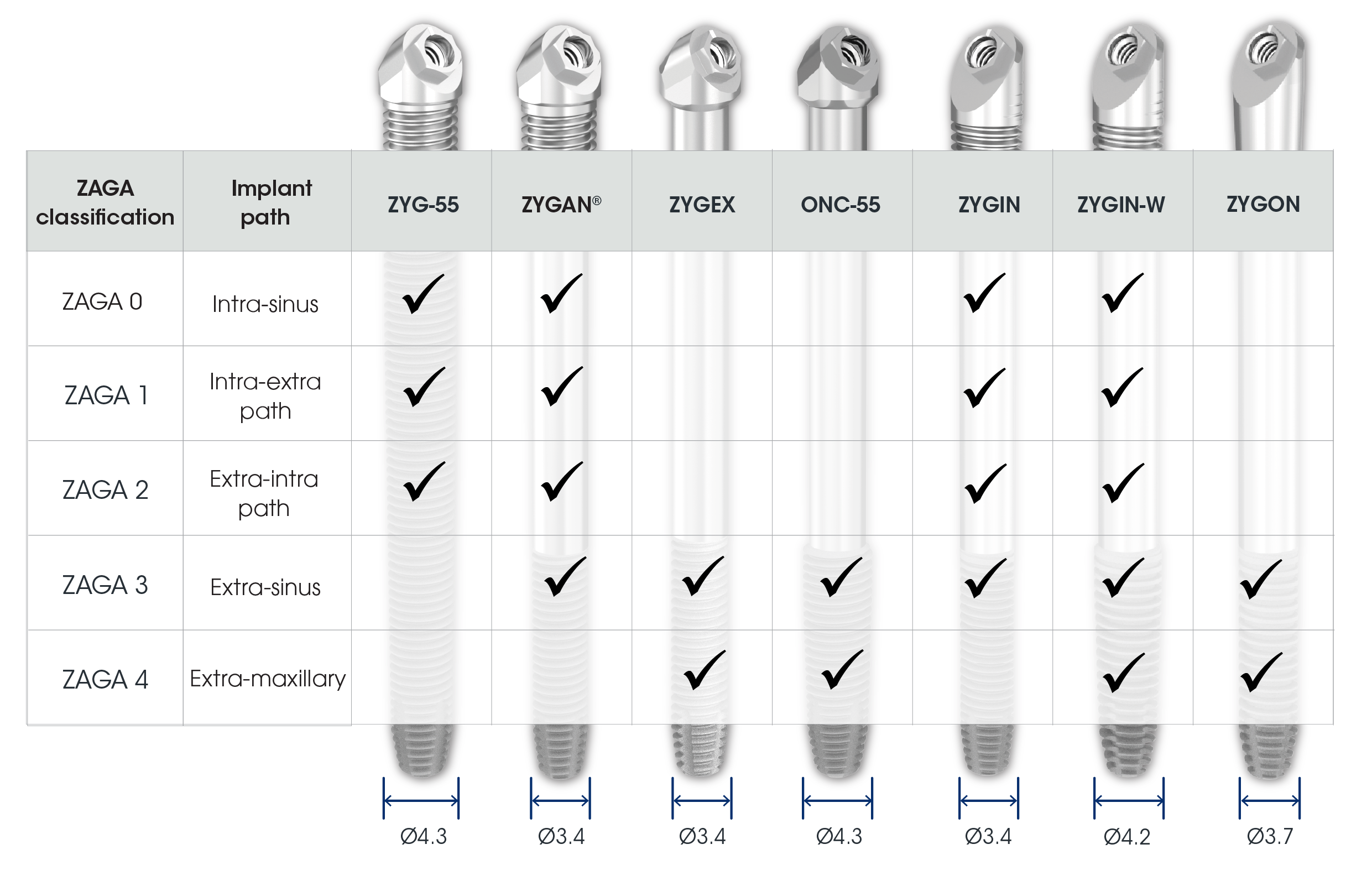
Implant variations to match the patient’s ZAGA classification and the surgeon’s placement technique
Technical Facts
- Available in lengths ranging from:
- Zygomatic implants: 30 – 60 mm
- Oncology implants: 27.5 – 47.5 mm
- Available in diameters:
- ZYGAN® and ZYGEX: ⌀3.4 mm apex
- ZYG-55 and ONC-55: ⌀4.1 mm
- Co-Axis® enabled: available in a 55° angulation
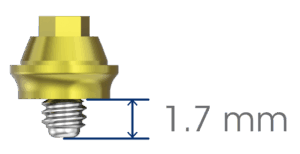
- Available with a MSC (Machined Surface Coronal) hybrid surface
- SInergy surface: surface roughened by alumina-blasting giving a moderately rough surface with over 20 years of evidence of clinical success
- Utilises ⌀4 mm prosthetics
Surgical Benefits
- Co-Axis® enabled: available in a 55° angulation
- Available with a MSC (Machined Surface Coronal) hybrid surface
- SInergy surface: surface roughened by alumina-blasting giving a moderately rough surface with over 20 years of evidence of clinical success
- The Oncology implant has a 15 mm threaded apex and a coronal machined surface that can be exposed to soft tissue in oncology resections
- The ZYGAN® implant features a narrow apex with a smooth mid-section and MSC threaded coronal region. The ZYGAN® is perfect for patients with smaller anatomy
- The ZYGEX implant offers the same machined area with a narrower apex of 3.4 mm (threaded body diameter)
- Pure high strength titanium enables exceptional fatigue strength (>920 MPa)
- Machined sections for exposed soft tissue and decreased rotations
Prosthetic Benefits
- Classic, trusted connections with sought-after modern features
- Forgiving interface for implant divergence
- Widest range of prosthetic options for treatment of partial or full edentulism
- 55° Co-Axis® provide the ideal prosthetic platform for screw-retained restorations

Catalogues and Brochures
Instructions For Use (IFU)
Documents
Videos and Animations
References
Boyes-Varley, J.G., Howes, D.G., Davidge-Pitts, K.D., Brånemark, P.I. and McAlpine, J.A., 2007. A protocol for maxillary reconstruction following oncology resection using zygomatic implants. International Journal of Prosthodontics, 20(5).
Boyes-Varley, J.G., Howes, D.G. and Lownie, J.F., 2003. The zygomaticus implant protocol in the treatment of the severely resorbed maxilla. SADJ: Journal of the South African Dental Association= Tydskrif van die Suid-afrikaanse Tandheelkundige Vereniging, 58(3), pp.106-9.
Chrcanovic, B.R., Albrektsson, T. and Wennerberg, A., 2016. Survival and complications of zygomatic implants: an updated systematic review. Journal of Oral and Maxillofacial Surgery, 74(10), pp.1949-1964.
Dattani, A., Richardson, D. and Butterworth, C.J., 2017. A novel report on the use of an oncology zygomatic implant-retained maxillary obturator in a paediatric patient. International Journal of Implant Dentistry, 3(1), pp.1-6.
Pellegrino, G., Tarsitano, A., Basile, F., Pizzigallo, A. and Marchetti, C., 2015. Computer-aided rehabilitation of maxillary oncological defects using zygomatic implants: a defect-based classification. Journal of Oral and Maxillofacial Surgery, 73(12), pp.2446-e1.
Pellegrino, G., Tarsitano, A., Basile, F., Pizzigallo, A. and Marchetti, C., 2015. Computer-aided rehabilitation of maxillary oncological defects using zygomatic implants: a defect-based classification. Journal of Oral and Maxillofacial Surgery, 73(12), pp.2446-e1.








